Slow Ion Collisions
|
In the case of slow ion collisions, the projectile velocity is much smaller than the velocity of bound electrons in the atom or molecule (vcollision ≤ velectron) and usually the collision energy varies between 1 keV and 100 keV, respectively. Due to this small collision velocity the target electrons are able to adjust their electronically states to the adiabatically changing core potentials; the electrons are “molecularized” between the projectile and the target. Here the most probable reaction channel is the charge transfer from one collision partner to the other. |
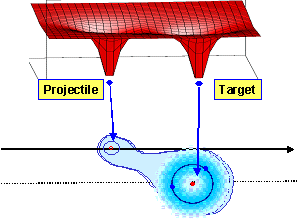 Saddle Point Ionization
Saddle Point Ionization Over Barrier Model
Over Barrier Model